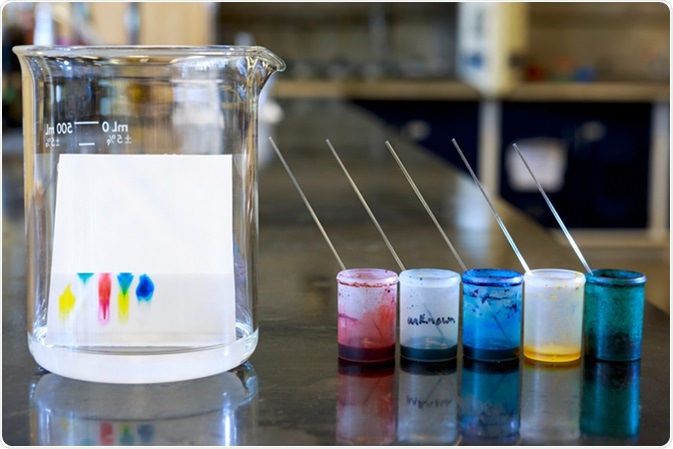
What is Chromatography? This technique was introduced in the 19th century and it is still in use. The term chromatography means color writing and it originated from a Greek word. In Greek, “chrome” means color, and “graphein” means to write.
Chromatography is an analytical technique used in the separation of a mixture of substances into its individual components with the help of a stationary phase and a mobile phase.
This technique also facilitates the purification of various compounds. Chromatographic methods highly rely on the use of the mobile phase and the stationary phase.
Mobile phase
This phase moves and transports the substances for separation onto the stationary phase. Liquids and gases are widely used in mobile phases. This phase is used as a carrier.
Stationary phase
This phase is immobile and still. During chromatography, the mobile phase passes through the stationary phase and deposits the substance leading to separation. This phase serves as a substrate. Common examples include paper, glass, alumina, and silica.
It is performed in biochemistry and molecular biology laboratories. In this article, we discuss chromatography in detail.
What is a chromatogram?
The chromatography process results in a chromatogram. A chromatogram is the visual representation of data generated, during the separation of substances in a chromatographic process. The data is generally represented in the form of graphs or plots.
Principle of chromatography
When the chemicals to be separated are carried by the mobile phase. The mobile phase passes through the substrate and the chemicals present in the mobile phase are adsorbed onto the surface of the stationary phase.
Adsorption is a result of various interactions that occur between the solute (chemicals to be separated) and the solid substrate. The degree of interaction and separation depends on the property of the substance that is being separated in the chromatographic process.
5 Types of chromatography
There are various types of chromatography and each chromatography is different and unique. The five main types of chromatography include:
- Paper chromatography
- Thin-layer chromatography
- Liquid chromatography
- Gas chromatography
- Ion exchange chromatography
- Paper chromatography
This is the most simple and common method of chromatography. In this method, a strip of paper is used as a stationary phase, and the solvent moves by capillary action. This capillary action helps to bring the solvent up the paper and facilitates the separation.
Applications
- Used in the separation of amino acids and antibodies
- Helps in antibiotic testing
- Used in forensic analysis for DNA fingerprinting.
- Thin-layer chromatography
It is a rapid and effective method of separation. In this method, adsorbent material like silica or alumina is coated on the thin glass or plastic plate and this serves as the solid phase for separation.
Applications
- Helps to estimate the purity of organic compounds
- Used in the textile industry and helps to determines dye composition in fabrics
- Used in the food industry to detect adulteration and pesticide residues.
- Liquid chromatography
In this technique, the liquid is used as the mobile phase. This chromatography is suitable for both qualitative and quantitative analysis. Any compound that is soluble in the liquid phase can be separated in liquid chromatography.
Applications
- Helps to determine the levels of contaminants and pollutants in environmental testing
- Used in water quality testing
- Used to characterize organic and inorganic compounds and biological samples.
- Gas chromatography
Gas chromatography is a powerful technique introduced in the 1950s. It is also known as gas-liquid chromatography. This method enables the separation of gases and volatile compounds. It is rapid and highly sensitive.
Applications
- Used in the pharmaceutical industry in the analysis of drugs and their metabolites.
- Helps to check the purity of various compounds
- Used in the toxicological analysis.
- Ion exchange chromatography
This technique is used to separate a mixture of similarly charged ions with the help of an ion exchange resin. It is an ideal method to separate polar molecules. This also enables the separation of cations and anions, depending on their affinity to the ion exchanger.
Applications
- In molecular biology, Used to separate proteins and nucleic acids
- Used to soften hard water and involved in the preparation of deionized water
- Helps to detect additives and contaminants in the food industry.
Advantages
Chromatography is highly beneficial and most preferred when compared to other techniques. Here’s a list of advantages offered by chromatographic methods:
- It is a fast and rapid method
- Enables the separation of complex mixtures of substances.
- Substances that have similar physical and chemical properties can also be separated.
- It has wide applications and used in various industries
- It offers high precision and accuracy.
- Very small quantities of samples are required for analysis.
- Chromatography facilitates the purification of samples.